One platform
Fully scalable
Backed by experts
- One screen for PIFs, CPSR, REACH, and regulatory compliance & safety
- Real-time insights into current and future compliance; 24/7 updated regulatory & tox data
- Direct access to senior Safety Assessors and Regulatory Specialists
- Optional EU/UK Responsible Person and US Agent services
- Auditable and traceable, with no AI guesswork involved
- Reduce costs by up to 40% by leveraging smart algorithms and workflows
start in 4-6 weeks
From spec data and documents to ready reports:
smart workflows, clear outcomes.
Faster, easier, current and future compliance management implemented in 4-6 weeks.
Ensure your product is built on insight, ready for market, and meets all safety and compliance standards.
+40%
savings through workflows and smart algorithms
5x
faster to prepare safety & compliance reports
5000+
products already managed in PRIMS
CORE FEATURES
Complete and up to date regulatory and toxicology data.
Plus the smart tools that simplify compliance.
Full specification management
Each raw material, formula, packaging component and product is managed as a single, connected item. You can update, version, clone and manage all item specific documentation. Ensuring consistency, traceability and full history.
Data & smart
algorithms
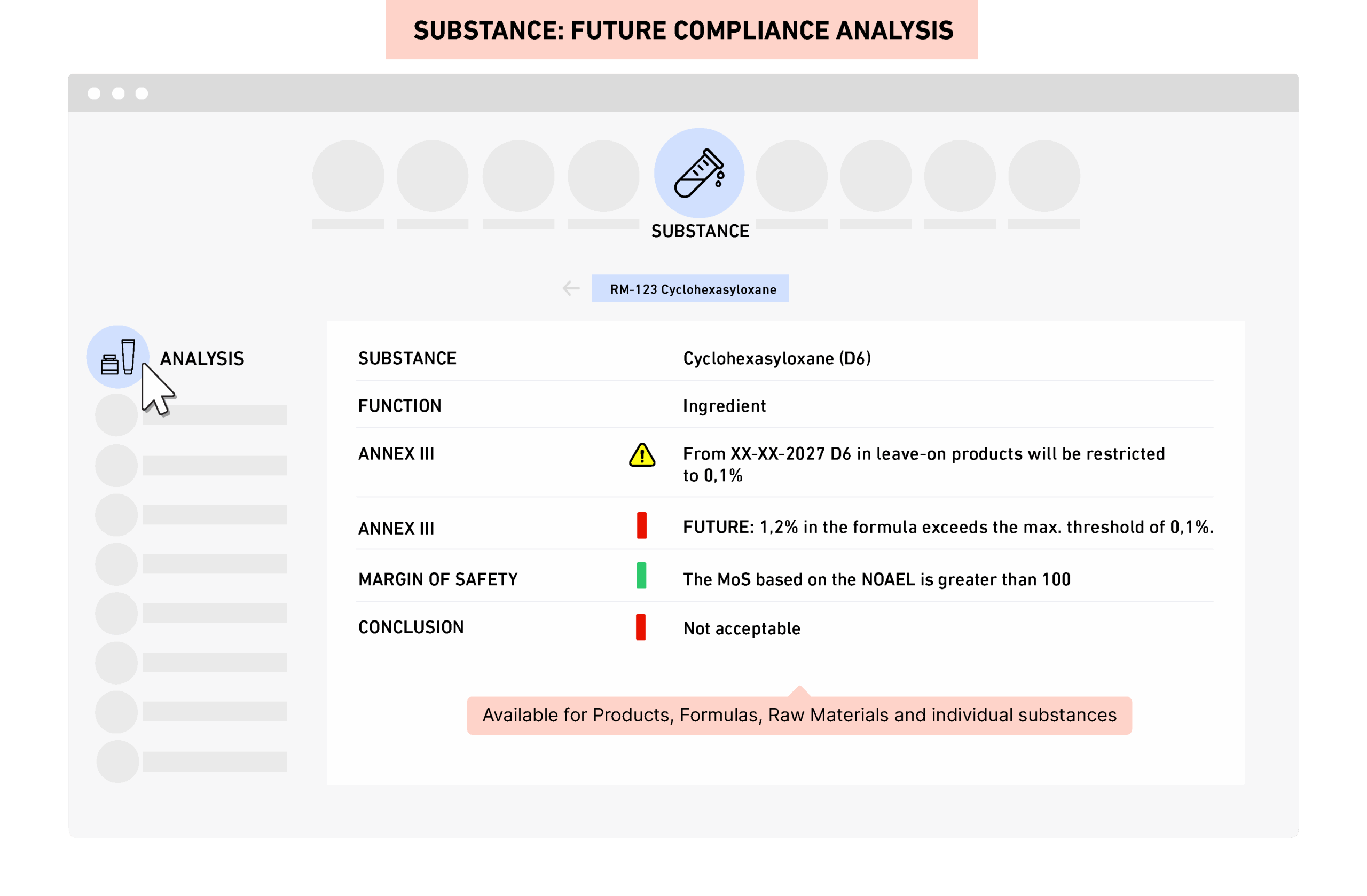
PRIMS algorithms link, connect, calculate and assess in real time against up-to-date and extensive regulatory, retailer and toxicology databases resulting in exact current & future yes/no compliance status and highlighting conditions and mandatory label content.
Dashboards & reports
Safety reports, ingredients lists, label content, Adverse Events, REACH management presented in dashboard format, and portfolio-wide where-used queries. All at your fingertips, and easily (re)generated and exported per product and for the full portfolio.
Clarity
from experts
PRIMS is developed by a team of toxicologists and assessors like you to be intuitive to navigate, and with full insight and overview to give you full clarity and control. And, when you need it, you get dedicated expert help including on-demand services.
Testimonials
PRIMS is developed and maintained by The Regulatory Company team – including Safety Assessors, Chemists, Toxicologists, PIF Managers and Regulatory Experts with extensive knowledge of the Personal Care Industry.
Through PRIMS, you gain direct access to expertise and tailored services that help you expand into new markets, manage complex portfolios, and attract new clients.
FAQ
Need help,
any questions?